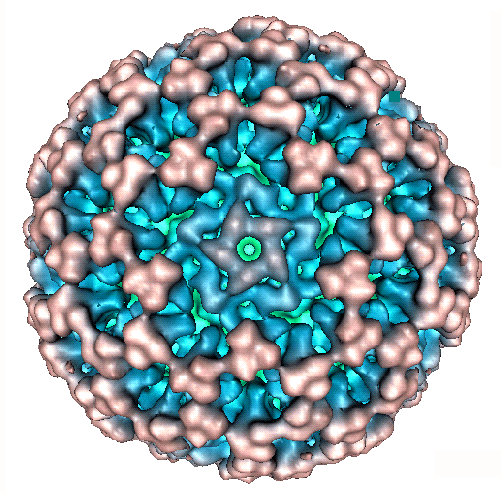
|
[ Up ]
The
dsRNA genome segments and proteins of Pseudomonas phage Phi 6
(genus Cystovirus : family Cystoviridae)
(available
as a Word file)
last updated 11/09/2002
|
dsRNA
(Size, bp)
[5,8,20,23] |
Open
reading frames (ORFs)
[5,8,20,23] |
Proteins
( ':
protein structure/ function) |
Size
'aa' (kDa )
[5,8,20,23] |
protein
copy number per particle |
location |
Function
|
|
L
(6,374) |
Gene
1 |
P1
(T=2) |
769
(84.986) |
120
[2.
6] |
inner
particle shell |
Self
assembles with proteins P2, P4 and P7 into procapsid particles [9, 29].
Corresponds to the T=2 particle in BTV [11]. Controls procapsid size and
organization. Binds minor proteins and RNA. Conserved in dsRNA viruses. |
|
L
|
Gene
2 |
P2
(pol)
[3,
18, 19] |
664
(74.791) |
12
[6] |
inner
particle |
Semi-conservative
RNA dependent RNA polymerase [34, 35]. Isolated protein acts on ss and
dsRNA templates [18, 19]. Three-dimensional structure homologous to HIV
reverse transcriptase and hepatitis C polymerase [3]. Binding sites for
nucleic acid, NTPs, Mn and Mg ions known [3]. |
|
M
(4,063) |
Gene
3 |
P3
(spike)
[17] |
648
(69.178) |
|
extends from
virion surface [17] and is attached to the integral membrane protein P6
[25] |
Binds
to the receptor [25] , the pilus IV of Pseudomonas syringae [30, 31] |
|
L
|
Gene
4 |
P4
(NTPase)
[7,
10, 16] |
331
(35.032) |
72
[7] |
inner
particle at 5 fold axes
[7] |
Hexameric
NTPase, symmetry mismatched position [7, 10, 16]. Powers the packaging
of ssRNA genomic precursors and is involved in transcription [27].
Nucleates the assembly of the procapsid with P1 [29] |
|
S
(2.948) |
Gene
5 |
P5
(mur)
[4,
12, 21] |
219
(24.018) |
|
between
the membrane and NC
[12] |
Muralytic
enzyme exposed to host peptidoglycan layer after membrane fusion during
viral entry. Also used to lyse the host cell late in infection to
release the virus [4, 12, 21]. |
|
M
|
Gene
6 |
P6
(fus)
[ 1] |
167
(17.229) |
|
Membrane
[32] |
Integral
membrane protein that has membrane fusion activity and binds the
receptor -binding protein, P3 [1, 25, 32]. |
|
L
|
Gene
7 |
P7
(assembly factor) [29] |
160
(17.169) |
60
[15] |
inner
particle
[15] |
Dimeric,
stabilizes the procapsid [15]. Increases the assembly rate of the
procapsid in vitro [29] |
|
S
|
Gene
8 |
P8
(T=13)
[2] |
148
(15.873) |
600
[2,
29] |
second
protein layer
[2] |
A
trimer [29] that forms the T=13 nucleocapsid shell around the
dsRNA-containing inner particle (core) [2]. During entry P8 drives the
penetration of the nucleocapsid into the cytoplasm [26, 28]. |
|
S
|
Gene
9 |
P9
(env)
[13,
32, 33] |
89
(9.482) |
|
Membrane
[32] |
Major
membrane protein, essential for membrane formation [13, 32, 33] |
|
M |
Gene
10 |
P10
(lys)
[14] |
42
(4.272) |
|
Membrane
[32] |
Needed
for cell lysis [14] |
|
S |
Gene
12 |
P12
(assembly)
[13.
22, 25] |
195
(20.293) |
0 |
Non-structural
[32] |
Assembly
factor active in viral membrane morphogenesis [13, 22, 25]. |
|
M |
Gene
13 |
P13 |
72
(7.649) |
|
Membrane
[8] |
Minor,
nonessential membrane protein [8, 24] |
|
L |
Gene
14 |
P14 |
61
(6.810) |
0 |
Non-
structural
[5] |
Nonessential
[5]. |
' :
': To facilitate comparisons of proteins across species, genera and families,
protein structure / function indicators have been added to protein names, eg (pol)
= RNA polymerase; (T=2) = capsid protein organized with icosahedral T=2
symmetry; (T=13) = capsid protein organized with icosahedral T=13 symmetry.
Constructed
by Sarah Butcher and Dennis Bamford.
If you
have any queries regarding this page please contact Peter Mertens on
peter.mertens@bbsrc.ac.uk.
References.
Bamford,
D.H., Romantschuk, M. and Somerharju, P.J. (1987) Membrane fusion in
prokaryotes: bacteriophage phi6 membrane fuses with the Pseudomonas syringae
outer membrane. EMBO J., 6, 1467-1473.
Butcher,
S.J., Dokland, T., Ojala, P.M., Bamford, D.H. and Fuller, S.D. (1997)
Intermediates in the assembly pathway of the double-stranded RNA virus f6. EMBO
J., 16, 4477-4487.
Butcher,
S.J., Grimes, J.M., Makeyev, E.V., Bamford, D.H. and Stuart, D.I. (2001) A
mechanism for initiating RNA-dependent RNA polymerization. Nature, 410, 235-40.
Caldentey,
J. and Bamford, D.H. (1992) The lytic enzyme of the Pseudomonas phage phi 6.
Purification and biochemical characterization. Biochim Biophys Acta, 1159,
44-50.
Casini,
G. and Revel, H.R. (1994) A new small low-abundant nonstructural protein encoded
by the L segment of the dsRNA bacteriophage phi 6. Virology, 203, 221-8.
Day,
L.A. and Mindich, L. (1980) The molecular weight of bacteriophage phi6 and its
nucleocapsid. Virology, 103, 376-385.
de
Haas, F., Paatero, A.O., Mindich, L., Bamford, D.H. and Fuller, S.D. (1999) A
symmetry mismatch at the site of RNA packaging in the polymerase complex of
dsRNA bacteriophage phi6. J Mol Biol, 294, 357-72.
Gottlieb,
P., Metzger, S., Romantschuk, M., Carton, J., Strassman, J., Bamford, D.H.,
Kalkkinen, N. and Mindich, L. (1988a) Nucleotide sequence of the middle dsRNA
segment of bacteriophage phi 6: placement of the genes of membrane-associated
proteins. Virology, 163, 183-90.
Gottlieb,
P., Strassman, J., Bamford, D. and Mindich, L. (1988b) Production of a
polyhedral particle in Escherichia coli from a cDNA copy of the large genome
segment of Phi 6. J. Virol., 62, 181-187.
Gottlieb,
P., Strassman, J. and Mindich, L. (1992) Protein P4 of the bacteriophage phi 6
procapsid has a nucleoside triphosphate-binding site with associated nucleoside
triphosphate phosphohydrolase activity. J. Virol., 66, 6220-2.
Grimes,
J.M., Burroughs, J.N., Gouet, P., Diprose, J.M., Malby, R., Zientara, S.,
Mertens, P.P. and Stuart, D.I. (1998) The atomic structure of the bluetongue
virus core. Nature, 395, 470-8.
Hantula,
J. and Bamford, D.H. (1988) Chemical crosslinking of bacteriophage ø6
nucleocapsid proteins. Virology, 165, 482-488.
Johnson,
M.D. and Mindich, L. (1994a) Plasmid-directed assembly of the lipid-containing
membrane of bacteriophage phi 6. J Bacteriol, 176, 4124-32.
Johnson,
M.D. (3rd) and Mindich, L. (1994b) Isolation and characterization of nonsense
mutations in gene 10 of bacteriophage phi 6. J. Virol., 68, 2331-8.
Juuti,
J.T. and Bamford, D.H. (1997) Protein P7 of phage phi6 RNA polymerase complex,
acquiring of RNA packaging activity by in vitro assembly of the purified protein
onto deficient particles. J Mol Biol, 266, 891-900.
Juuti,
J.T., Bamford, D.H., Tuma, R. and Thomas, G.J., Jr. (1998) Structure and NTPase
activity of the RNA-translocating protein (P4) of bacteriophage phi 6. J Mol
Biol, 279, 347-59.
Kenney,
J.M., Hantula, J., Fuller, S.D., Mindich, L., Ojala, P.M. and Bamford, D.H.
(1992) Bacteriophage phi 6 envelope elucidated by chemical cross-linking,
immunodetection, and cryoelectron microscopy. Virology, 190, 635-644.
Makeyev,
E.V. and Bamford, D.H. (2000a) The polymerase subunit of a dsRNA virus plays a
central role in the regulation of viral RNA metabolism. EMBO J., 19, 6275-6284.
Makeyev,
E.V. and Bamford, D.H. (2000b) Replicase activity of purified recombinant
protein P2 of double- stranded RNA bacteriophage phi6. EMBO J, 19, 124-133.
McGraw,
T., Mindich, L. and Frangione, B. (1986) Nucleotide sequence of the small
double-stranded RNA segment of bacteriophage phi 6: novel mechanism of natural
translational control. J. Virol., 58, 142-151.
Mindich,
L. and Lehman, J.F. (1979) Cell wall lysin as a component of the bacteriophage
ø6 virion. J. Virology, 30, 489-496.
Mindich,
L. and Lehman, J.F. (1983) Characterization of phi6 mutants that are temperature
sensitive in the morphogenetic protein P12. Virology,127, 438-445.
Mindich,
L., Nemhauser, I., Gottlieb, P., Romantschuk, M., Carton, J., Frucht, S.,
Strassman, J., Bamford, D.H. and Kalkkinen, N. (1988) Nucleotide sequence of the
large double-stranded RNA segment of bacteriophage phi 6: genes specifying the
viral replicase and transcriptase. J.Virol., 62, 1180-5.
Mindich,
L., Qiao, X., Onodera, S., Gottlieb, P. and Strassman, J. (1992) Heterologous
recombination in the double-stranded RNA bacteriophage phi 6. J. Virol., 66,
2605-10.
Mindich,
L., Sinclair, J.F. and Cohen, J. (1976) The morphogenesis of bacteriophage phi6:
particles formed by nonsense mutants. Virology, 75,224-231.
Olkkonen,
V.M., Ojala, P.M. and Bamford, D.H. (1991) Generation of infectious
nucleocapsids by in vitro assembly of the shell protein on to the polymerase
complex of the dsRNA bacteriophage phi 6. J Mol Biol, 218, 569-581.
Pirttimaa,
M.J., Paatero, A.O., Frilander, M.J. and Bamford, D.H. (2002) Nonspecific
nucleoside triphosphatase P4 of double-stranded RNA bacteriophage phi6 is
required for single-stranded RNA packaging and transcription. J Virol, 76.
Poranen,
M.M., Daugelavicius, R., Ojala, P.M., Hess, M.W. and Bamford, D.H. (1999) A
novel virus-host cell membrane interaction. Membrane voltage-dependent endocytic-like
entry of bacteriophage phi6 nucleocapsid. J Cell Biol, 147, 671-82.
Poranen,
M.M., Paatero, A.O., Tuma, R. and Bamford, D.H. (2001) Self assembly of a viral
molecular machine from purified protein and RNA constituents. Molecular Cell, 7,
845-854.
Roine,
E., Raineri, D.M., Romantschuk, M., Wilson, M. and Nunn, D.N. (1998)
Characterization of type IV pilus genes in Pseudomonas syringae pv. tomato
DC3000. Mol Plant Microbe Interact, 11, 1048-56.
Romantschuk,
M. and Bamford, D.H. (1985) Function of pili in bacteriophage phi6 penetration.
J. Gen. Virol., 66, 2461-2469.
Sinclair,
J.F., Tzagoloff, A., Levine, D. and Mindich, L. (1975) Proteins of the
bacteriophage phi6. Virology, 75, 685-695.
Stitt,
B.L. and Mindich, L. (1983) Morphogenesis of bacteriophage phi6: a presumptive
viral membrane precursor. Virology, 127, 446-458.
Usala,
S.J., Brownstein, B.H. and Haselkorn, R. (1980) Displacement of parental RNA
strands during in vitro transcription by bacteriophage phi6 nucleocapsids. Cell,
19, 855-862.
Van
Etten, J.L., Burbank, D.E., Cuppels, D.A., Lane, L.C. and Vidaver, A.K. (1980)
Semiconservative synthesis of single-stranded RNA by bacteriophage phi6
polymerase. J. Virol., 33, 769-773.

|