 |
ReoID Phylogenetic sequence analysis and improved diagnostic assay systems for viruses of the family Reoviridae
Edited by Peter. P. C. Mertens and Houssam Attoui |
 |
| ||||||
|
Reoviridae and Orbivirus Classification (Introductory talk, Thessaloniki June 7th 2001: P. Mertens (Co-ordinator))
The members of the family Reoviridae are distinguished primarily by their genome, composed of 10, 11 or 12 linear segments of dsRNA. This large family of viruses includes nine established genera and two proposed new genera of viruses, many of which cause economically, or medically important diseases, affecting a wide range of plant and animal hosts (tables 1 and 2) (Mertens et al 2000). ICTV have now recognised one of the newly proposed genera Seadornaviruses but wish to find a new name for the proposed genus Entomoreovirus. There are also >5 unclassified members of the family that infect arthropods, suggesting possible requirements for still more new genera (table 1) (Mertens et al 2000).
* Based on sequence comparisons, it has been proposed that the genus Coltivirus should be split into two genera, Coltivirus and Seadornavirus (a new genus).
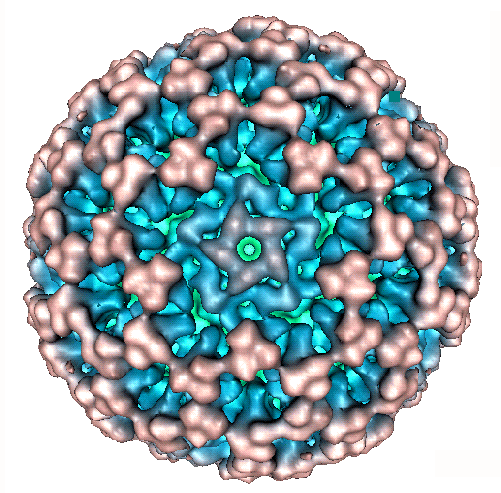
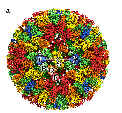
In the recent 7th taxonomy report for ICTV, the ability of viruses to reassort genome segments was recognised as a primary determinant of virus species within the family Reoviridae (Mertens et al 2000). This reflects the difficulties that would exist in separately classifying each different reassortant virus that could be generated from even two distinct parental virus strains containing 10-12 different genome segments each. The ability to reassort genome segments and to generate viable progeny strains, clearly demonstrates a significant level of functional homology between the two parental virus strains and many studies have shown that this is reflected in significant serological and nucleotide sequence similarities. As a consequence both serological methods and sequence comparisons of appropriate, ‘conserved’ or ‘variable’ genome segments / proteins can be used to compare these different viruses and to identify members of individual genera, species and serotypes.In the past most of the effective methods for identifying different viruses and virus species within the Reoviridae were by means of serology, comparing the reaction of virus antigens with reference antisera, or the reaction of antibodies raised against the virus with reference antigens. However, these methods can be time consuming and rely heavily on the availability of high quality, standardised and therefore expensive serological reagents / assays. The lack of any laboratories that has the full range of these reagents to identify any new virus has lead to delays in the identification and assignment of new virus isolates (hence the large number of Orbivirus isolates that remain unidentified). However, in the 7th ICTV report on virus taxonomy, phylogenetic sequence analyses were specifically recognised as one of the ‘parameters’ that can be used to define virus species within the family Reoviridae (Mertens et al 2000). In effect both serological methods and sequence analyses can be used to predict the compatibility of these viruses for genome segment reassortment and therefore to identify different virus species.One major advantage of sequencing methods is that comparisons to earlier sequence data can be carried out over the Web, abolishing the requirement for standardised reagents. However, such comparisons are only possible if sufficient data has already been generated for known virus isolates to allow meaningful comparisons. One of the major objectives of the REO ID project is therefore to generate additional data, making it possible to identify more ‘new’ virus isolates simply by sequence comparisons.The members of the genus Orbivirus were previously divided into ‘serogroups’ based on their serological cross-reactions in complement fixation (CF), agar gel immunodiffusion (AGID), or fluorescent antibody tests. These ‘serogroups’ correlate with the different Orbivirus species that are now recognised (Table 1 and 2). More recently enzyme linked immunosorbent assays (ELISA) and PCR based assays have been used as diagnostic tests to detect and identify some of the better studied viruses, for example members of the BTV species. These assays, which detect BTV specific antigens, antibodies, or nucleic acid are accepted internationally for testing of animals for import / export and are now used, more than other methods, to detect and identify BTV. The major species (serogroup) specific antigen of BTV that is recognised in these assays is the outer core protein VP7 (T13) (figure 1).
Figure 1: Model of the native BTV core particle from X-ray crystallography (Grimes et al, 1998). The outer core surface is composed of 260 trimers of VP7(T13) The major BTV species specific antigen, arranged with T = 13 l symmetry. The chemically identical but structurally different trimers are coloured and named in order of increasing distance from the five fold axes of symmetry (P-red, Q-orange, R-yellow, S-green and T-blue situated at the three fold axes). Sequence data now exists for at least some genome segments of representative isolates from each of the genera within the Reoviridae and in an increasing number of cases this includes full genome data (for example the genome of Cypovirus 1 and an Aquareovirus were recently completed). Data concerning the proteins and RNAs of these viruses is available via the dsRNA virus web pages at http://www.reoviridae.org/dsRNA_virus_proteins/ This site will be supported and expanded as part of the Reo ID project to include more data. The genus Orbivirus contains a total of 20 established virus species, although sequence data is not yet available for representatives of many of them (table 2). As a consequence although it is possible to identify new viruses as a members of specific genera, (for example as an Orbivirus) by analyses and comparisons of conserved genome segments (e.g. genome segment 1, coding for the polymerase: figure 2), it is not yet possible to assign these new isolates to specific Orbivirus species by such methods. This explains why the genus Orbivirus currently includes 11 unassigned viruses.
Figure 2: Phylogenetic tree of the amino acid sequences of the polymerase protein of some representative members of the different genera of the family Reoviridae.
Phylogenetic tree for the Reoviridae RNA polymerase. Amino acid sequences derived from the nucleotide sequence of the relevant genome segment (segment 1 in each case, except for genome segment 4 of rice ragged stunt virus - Oryzavirus ), were aligned by using the CLUSTALW 1.60 program (Higgins & Sharp, 1989). The Neighbour-joining tree was prepared using Clustal X (Thompson et al., 1997), allowing for multiple substitutions and ignoring gaps and drawn with TreeView 1.5 (Page, 1996). Strains of viruses and their sequences used: Orthoreovirus - Mammalian orthoreovirus subgroup 1, serotype Dearing 3 (MRV-3) M24734; Orbivirus - African horse sickness virus serotype 9 (AHSV-9) U94887; bluetongue virus, serotype 2 (BTV-2) L20508; bluetongue virus, serotype 10 (BTV-10) X12819; bluetongue virus, serotype 11 (BTV-11) L20445; bluetongue virus, serotype 13 (BTV-13) L20446; bluetongue virus, serotype 17 (BTV-17) L20447. Rotavirus - bovine rotavirus, Group A (A-BoRV) J04346; simian rotavirus, Group A (SA11 A-SiRV) AF015955; murine rotavirus IDIR, Group B (B-MuRV) M97203; porcine rotavirus, Group C (C-PoRV) M74216; Fijivirus - Nilaparvata lugens reovirus Izumo strain (NLRV) D49693; Phytoreovirus - rice dwarf virus, Chinese strain (RDV) U73201; rice dwarf virus, strain H (RDV) D10222; rice dwarf virus (RDV) D90198; Oryzavirus - rice ragged stunt virus, Thai strain (RRSV) U66714. No polymerase sequences have either been identified or are currently available for any cypoviruses, coltiviruses, aquareoviruses or any of the unclassified viruses of invertebrates. The Reo ID project will seek to provide sequence data for representative conserved genome segments (e.g. genome segment 3 encoding the conserved VP3 (T2) protein see figures 3 and 4) for isolates of each of the Orbivirus species and for the 11 ‘new’ orbiviruses that are currently unassigned. The power of this approach has already been demonstrated by the analysis (at Marseilles) of Ndelle virus (manuscript in preparation), previously classified as an Orbivirus but now reassigned as an Orthoreovirus and the recognition that St Croix River virus is a (20th) new Orbivirus species (Attoui et al 2001).
Figure 3: Phylogenetic tree for the orbiviruses, constructed using partial genome segment 3 (nucleotides 1193-1661), or equivalent sequences (encoding the major structural protein of the VP3(T2) subcore capsid shell). Nucleotide sequences were aligned by using the CLUSTALW 1.60 program Higgins and Sharp (1989). The tree was prepared using Clustal X, Thompson et al., (1997) and drawn with TreeView 1.5 (Page, 1996).Viruses and accession numbers used were: PALYAM (D'Aguilar B8112), WALLAL (Ch 12048), WARREGO (Ch 9935), CORRIPARTA (CSIRO 109) and EUBENANGEE (In1074) Pritchard et al, (1995); v595 and CS131 Gould and Parkes (1996); v654, v370 are recent corriparta serogroup isolates, Warrego-K and Wallal-K are kangaroo isolates from a recent outbreak of chorioretinitis in western grey kangaroos Hooper et al (1998); BTV1SA; BTV3SA; BTV4SA; BTV9SA are South Africa vaccine strains and BTV15SA was a South Africa isolate, BTV1Honduras, BTV3Guatemala, BTV4Dominican Rep, BTV6Honduras, BTV8Dominican Rep and BTV12Jamaica were Caribbean isolates (Pritchard et al, 1995) ; EHDV2Aus (S68010); EHDV2USA (L33820); EHDV1USA (X61589); AHSV4 (D26572); Broadhaven virus (M87875); WONGOR (U56992); Paroo River (U56993); Picola (U56994); v195 (U56990); v199 (U56991); v1447 (U56989); BTV3Aus (L26566), BTV9Aus (L26565), BTV15Aus, BTV16Aus (L26557), BTV20Aus (L26563), BTV21Aus (L26564), BTV23Aus (L26568), BTV1Malaysia (L26560), BTV9Indonesia (L26562), BTV16Indonesia (L26558), BTV23Indonesia (L26567), BTV1India (L26559), BTV2 India (L26561). BTV2USA (S78452). BTV10USA (M22096); BTV11USA (L19968), BTV13USA (L19969) and BTV17USA (K02369).
Figure 4: Model The BTV1 subcore shell from X-ray crystallography of the native core particle (Grimes et al 1998). The subcore is composed of 120 copies of the very highly conserved VP3(T2) protein, arranged with T = 2 symmetry. The chemically identical but structurally different 'A' (green) and 'B' (red) molecules are shown. Each of the Orbivirus species (Table 2) also contains a number of distinct serotypes that can be distinguished in serum neutralisation assays, for example Bluetongue virus (BTV), the type species of the genus, containing a total of 24 distinct serotypes. Although these serotypes are not recognised in the formal virus taxonomy, they are of very real biological significance, since the virus serotype has direct relevance to serological protection and allows the identification of appropriate virus strains/antigens for use in vaccination campaigns. BTV serotype is determined by the most variable outer capsid proteins VP2 and VP5, encoded by genome segments 2 and 6 respectively. In consequence the sequence of BTV genome segment 2 and to a lesser extent genome segment 6 correlates with virus serotype.
Table 2. Viruses of the genus Orbivirus a, by serogroup (species), serotypes, hosts and principal vectors.
Sequence data are available for genome segment 2 some BTV serotypes and these can be used to construct a phylogenetic tree, Nick Knowles and Alan Samuel will present additional data for the conserved and variable proteins in the following talks. The Reo ID project will seek to provide representative sequence data for all of the BTV serotypes, in volving particularly the laboratories at Pirbright and at Maisons Alfort. This will help us to develop PCR based assays to identify the different BTV virus serotypes. However we need to be aware that there are many isolates of the different BTV serotypes around the world (see table 3), which although antigenically similar have significant levels of diversity in the sequence of their genome segment 2. Part of this process will therefore involve the design and testing / validation of primers and protocols for amplification of selected regions of segment 2 from different virus isolates of each serotype. It is therefore an important part of the project to establish and maintain a reference collection of BTV and other orbiviruses for these studies.
Figure 5: Phylogenetic tree for the orbiviruses constructed using partial VP2 sequences (amino acids 303-464). VP2 is the larger outer capsid protein and major neutralisation antigen. Amino acid sequences derived from the nucleotide sequence of genome segment 2 were aligned by using the CLUSTALW 1.60 program (Higgins & Sharp, 1989). The tree was prepared using Clustal X (Thompson et al., 1997) and drawn with TreeView 1.5 (Page, 1996). Viruses and accession numbers used were EHDV-2 (US), EHDV-2 (Aus) (Gould & Pritchard, 1991); BTV-3 (SA), BTV-15 (Aus), BTV-20 (Aus) Pritchard & Gould (1995); EHDV-1 (US) (D10767); AHSV-3 (U01832); AHSV-4 (M94680); BTV-1 (Aus) (X55800); BTV-3 (Aus) (L42168), BTV-9 (Aus) (L46686), BTV-16 (Aus) (L46683), BTV-21 (Aus) (L46684), BTV-23 (Aus) (L46685), BTV-11 (US) (M17437), BTV-13 (US) (D00153), BTV-17 (US) (M17438); BTV-2 (US) (M21946) BTV-1 (SA), BTV-10 (US) (M11787).
Table 3: The geographical distribution of different BTV serotypes (2001)
* The BTV serotypes from Europe have isolated from relatively short lived (up to 4 years) epizootics and the virus has subsequently been eradicated on each occasion.
One particularly significant aspect of these comparative analyses, particularly of genome segment 2, is their value in studying the epidemiology of these viruses, for example of BTV during the current out breaks around the Mediterranean in southern Europe, Turkey and north Africa. I had originally intended that Philip Mellor, who runs the Entomology section at Pirbright and who has a specific interest in the epidemiology of BTV would present an update concerning BTV particularly in the Western Mediterranean region. In his absence I will present a second talk tomorrow showing his epidemiological data. And try to explain how our sequencing studies may help to clarify what has happened and examine the potential threat posed by the use of live / attenuated BTV vaccines.
The Epidemiological Aspects of the project are also planned to include surveys of human sera from the blood bank in Marseilles, for antibodies to Coltiviruses. This will help to establish the distribution and spread of these viruses in the Human population in that part of Europe . The Project is also intended to generate additional information concerning the Aqareoviruses (at Madrid) and to provide epidemiological data on the spread incidence and impact of these viruses within Europe. These edidemiological studies (including studies of the orbiviruses) will also be supported by serological assays, including methods to be developed as part of the project.
References
Grimes J. M., Burroughs, J. N., Gouet, P., Diprose J. M., Malby. R., Zientara, S., Mertens, P. P. C. and Stuart, D. I. (1998) The atomic structure of the bluetongue virus core. Nature 395, 470-478.
Mertens, P. P. C., Arella, M., Attoui, H., Belloncik, S., Bergoin, M., Boccardo, G., Booth, T. F., Chiu, W., Diprose, J. M., Duncan, R., Estes, M. K., Gorziglia, M., Gouet, P., Gould, A. R., Grimes, J. M., Hewat, E., Hill, C.,Holmes, I. H., Hoshino, Y., Joklik, W. K., Knowles, N., López Ferber, M.L., Malby, R., Marzachi, C., McCrae, M. A., Milne, R. G., Nibert, M., Nunn, M., Omura, T., Prasad, B. V. V., Pritchard, I., Samal, S. K., Schoehn, G., Shikata, E., Stoltz, D. B., Stuart, D. I., Suzuki, N., Upadhyaya, N., Uyeda, I., Waterhouse, P., Williams, C.F., Winton, J. R. and Zhou, H. Z. (2000). Reoviridae. In "Virus Taxonomy. Seventh Report of the International Committee for the Taxonomy of Viruses".Eds. Van Regenmortel, M. H. V., Fauquet, C. M., Bishop, D. H. L., Calisher, C. H., Carsten, E. B., Estes, M. K., Lemon, S.M., Maniloff, J., Mayo, M. A., McGeoch, D. J., Pringle, C. R. and Wickner, R. B. Academic Press, pp 395- 480.
Attoui, H. Stirling, J. M.,. Munderloh, U. G., Billoir, F., Brookes, S. M., Burroughs, J. N., de Micco , P., Mertens, P. P. C. and de Lamballerie, X. (2001) Complete sequence characterisation of the genome of the St. Croix River Virus, a new orbivirus isolated from Ixodes scapularis cells. Journal of General Virology 82, 795-804.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|